The Capital Region Has This Many FDA-Registered Medical Device Establishments
The Capital Region has more than 20 U.S. Food and Drug Administration (FDA)-registered establishments involved in the manufacturing and preparation of more than 1,300 medical devices, according to a Center for Economic Growth (CEG) analysis of the FDA data.
Establishment Types
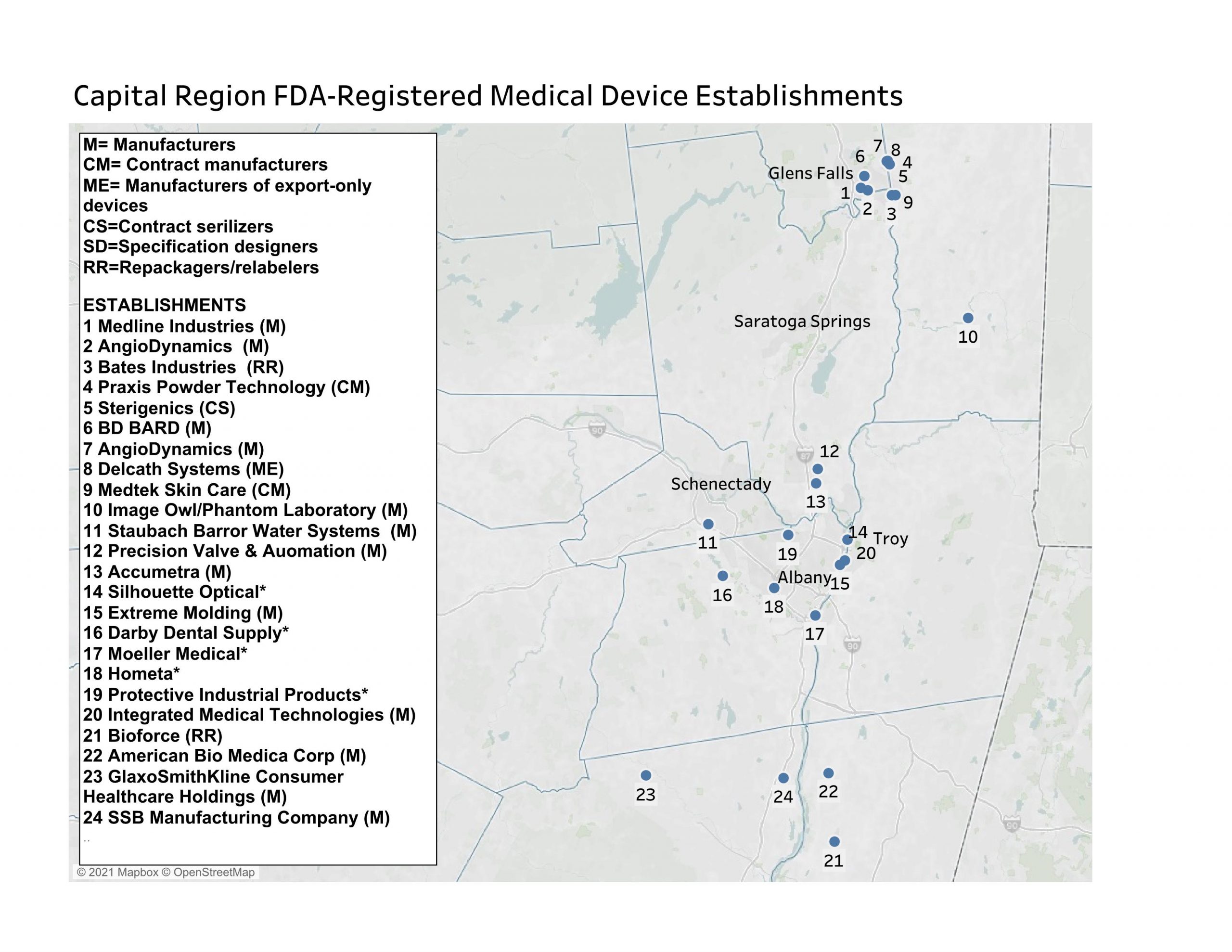
As of March 2021, the eight-county region had 25 FDA medical device establishments. Among them, 10 were involved in the manufacturing of medical devices, six in contract manufacturing, and five in the domestic manufacture of export-only devices. Three more establishments are specification developers and there is one contract sanitizer and one repackager/relabeler. Four establishments house initial distributor/importer offices but do not have any on-site production or preparation operations.
 Manufacturers
Manufacturers
The region’s establishments involved in any type of manufacturing have 545 medical devices registered for their sites. Manufacturers with the most medical devices registered for local establishments included AngioDynamics, with production facilities in Glens Falls (95 devices) and Queensbury (115 devices); American Bio Medica in Kinderhook (117); BD Bard in Queensbury (71); and Medline Industries in Glens Falls (30 devices).
While having smaller numbers of medical devices registered to their establishments, several local are noteworthy. For example, Precision Valve and Automation in Halfmoon last year received emergency use authorization from the FDA for its emergency resuscitator. Medtek Skin Care (dba UVBiotek) in Hudson Falls makes dermatological ultraviolet lights, and SSB Manufacturing makes mattress covers for hospitals.
Non-Registered Establishments
Only establishments that produce finished medical devices have to be registered with the FDA. The region has several other major facilities that are in the medical device supply chain. For example, GE Healthcare’s North Greenbush facility makes digital X-Ray detectors, and Philips Healthcare Solutions’ Latham plant makes magnets for MRI machines. Specialty Silicone Products in Ballston Spa makes ultra-clean silicones for medical devices and equipment.
Employment
The medical equipment and supplies manufacturing industry employs hundreds of jobs in the Capital Region. There is an exceptionally high concentration of these jobs in Warren County, which houses two AngioDynamics plants, Medline Industries, BD Bard, Sterigenics, Delcath Systems, Bates Industries, and Praxis Technology. Warren County has the nation’s highest concentration of medical equipment and supplies manufacturing industry, as measured by its location quotient. As of Q3 2020, this industry employed 1,496.
CEG Initiatives
CEG is supporting the growth of the region’s life sciences cluster by engaging in the following activities:
Marketing the Capital Region’s life sciences R&D assets at talent pipeline.
Conducting a life sciences cluster study that developed an action plan for: 1. improving the commercialization of local life sciences innovations, 2. strengthening the region’s life sciences ecosystem; 3. and recruiting contract research organizations (CROs) to the region.
Assisting life sciences startups with accessing labs, office space, and other facilities; developing venture pitches; identifying potential investors and mentors.
Helping biotech firms, such as Vital Vio and Precision Valve & Automation grow through Business Growth Solutions services, including continuous improvement, technology acceleration, energy and sustainability, supply chain development and workforce initiatives.
Don’t miss these insights into the trends that are shaping the Capital Region’s economy. Sign up for CEG’s e-news and follow us on:













